Wang Donghai, Academician Sun Xiuzhi, Kansas State University: bioprinted peptide hydrogel without light, chemical or ion crosslinker!
[guide to the main picture].

Figure 1.
Peptide hydrogels (PGmatrix and PGmatrix-M) have adjustable properties.
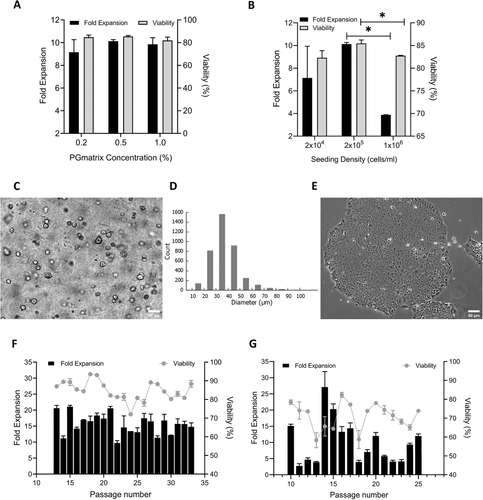
Figure 2.
Compared with the traditional 2D method, PGmatrix hydrogel shows superior hiPSC maintenance.
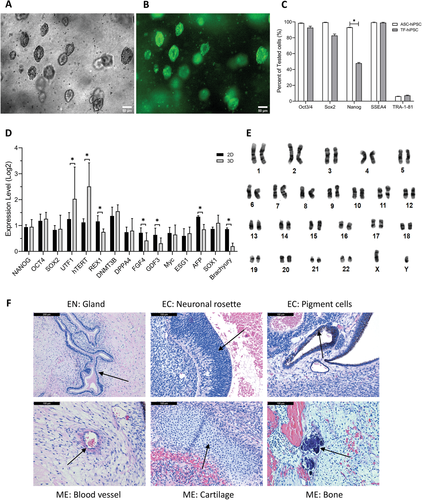
Figure 3.
ASC-hiPSCs maintained pluripotency during medium-and long-term culture of 3D PGmatrix hydrogel.

Figure 4.
Compared with 3D PEG-based hydrogels, hiPSC in 3D PGmatrix hydrogels shows excellent properties. A) ASC-hiPSCs grows better in 3D PGmatrix (0.5%) containing mTESR1 (0.5 PG-mT) than in 3D Mebiol gel containing E8 medium (Mebiol-E8).
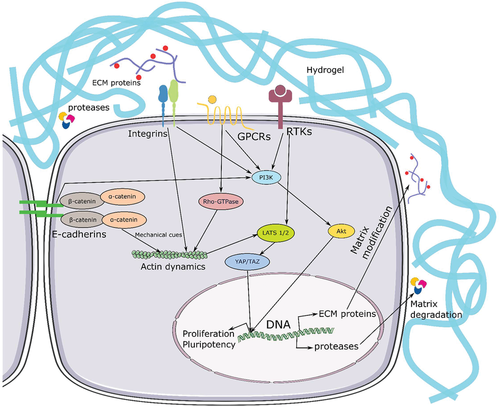
Figure 1 proposed mechanism for hiPSC growth and maintenance of pluripotency in 3D PGmatrix hydrogels.
Cells actively change the surrounding 3D microenvironment by secreting proteases as matrix degradation enzymes and ECM proteins. These modifications promote cell migration and signal transduction through cell-to-cell contact (E-cadherin) and paracrine signal molecules. In addition to soluble factor signals, physical (mechanical strength) cues from cell-matrix or cell-cell adhesion affect actin-myosin cytoskeleton and send signals through Hippo pathway, which ultimately affect the expression of mechanically sensitive YAP/TAZ gene to regulate and maintain the proliferation and pluripotency of hiPSC, so as to achieve long-term maintenance of hiPSC. GPCRs--G protein-coupled receptor and RTKs-- receptor tyrosine kinase.
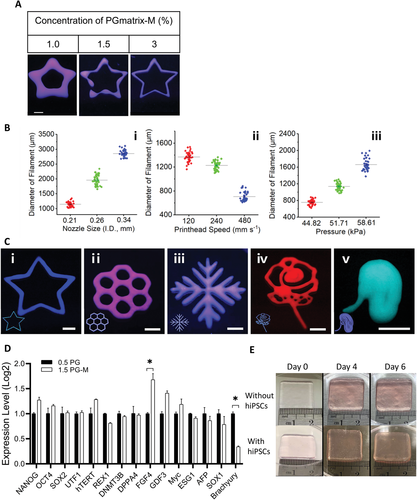
Figure 5.
PGmatrix-M as a biological ink printability and hiPSCs performance. A) the printing resolution of PGmatrix-M at different concentrations. B) I) nozzle size, ii) printhead movement speed and iii) influence of print pressure on PGmatrix-M bioink printability. C) Bio-printing PGmatrix-M structure of different patterns (3%). Scale. Five millimeters. D) ASC-hiPSC cultured in 1.5 PGmatrix-M (1.5 PG-M) showed no major difference in expression patterns of pluripotency related genes compared with those cultured in 0.5 PGmatrix (0.5 PG). E) the PGmatrix-M construct remains stable for at least 6 days with or without hiPSCs (destroy the construct before the 6th day to collect cells for further analysis).
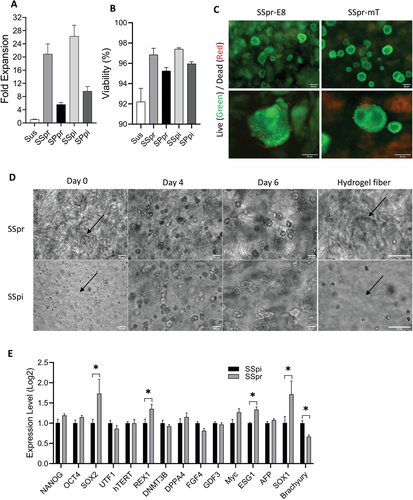
Figure 6.
The biological printing process did not significantly affect the growth performance of ASC-hiPSC encapsulated in PGmatrix-M bioink hydrogel.
[summary].
The best 3D culture conditions and adjustable universal peptide hydrogel (PGmatrix-M) for efficient physiological formation of hiPSC spheres have been developed with consistent cell growth (15-25 times), high cell viability of more than 95% and stable genetic integrity. Within the test range the growth performance of hiPSCs has nothing to do with the mechanical (i.e. strength and viscosity) and morphological (i.e. pore size) properties of PGmatrix or PGmatrix-M. Both PGmatrix and PGmatrix-M are high-quality scaffold hydrogels for self-renewal cells such as hiPSCs 3D culture (single cells or spheres) and long-term maintenance; however, PGmatrix-M exhibits large-scale gel strength, high viscosity and rapid self-healing kinetics, which is suitable for the physiological formation of hiPSCs spheres by fluid transfer or biological printing, without the need for light, chemical or ion crosslinkers. The survival rate of hiPSC after extrusion bio-printing is 96%, and the cell viability is higher than 94% at 0 and 24 hours, indicating that PGmatrix-M provides the greatest protection for hiPSC in the printing process. PGmatrix-M has the potential of hiPSC and can be used for industrial-scale single-cell or sphere manufacturing in downstream applications. This study shows that the gel degradation ability of cells is strongly beneficial to the growth of hiPSC, and it is speculated that this relationship is related to Hippo signal pathway and mechanically sensitive YAP/TAZ pathway. Whether peptide hydrogel is a chemical or biological clue provided by PGmatrix or PGmatrix-M can also be attributed to the growth of hiPSC remains to be studied.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
+86-18915413828(WhatsApp&WeChat)
Previous: Review of Liu Zongwen,


