Advanced Functional Materials:Ti3C2Tx nanowires and hydrated ammonium vanadate nanoribbons are flexible anodes for waterborne zinc ion batteries.
I. Overview of the article.
The development of zinc-free metal anode materials is an effective strategy to solve the problem of zinc metal dendrite which seriously hinders the development of zinc ion batteries. However, the research of zinc metal-free anode materials is still in its infancy, and more importantly, its practical application is seriously limited by its low energy density. In this paper, a new type of (NH4) 2V10O25 ·8H2O @ Ti3C2Tx (NHVO@Ti3C2Tx) thin film anode is proposed and studied, which is used to construct rocking chair ZIB. The capacity of NHVO@Ti3C2Tx electrode is 514.7mAhg-1, and the low potential is 0.59V (vs Zn2+/Zn) at 0.1Ag-1. The introduction of Ti3C2Tx not only provides an interconnected conductive network, but also stabilizes the structure of NHVO nanoribbons with a longer cycle life (84.2% after 6000 cycles of 5.0A g-1). As a proof of concept, a zinc-free full battery has been successfully demonstrated with a maximum capacity of 131.7 mAhg-1 (including anode and cathode mass) and an energy density of 97.1Whkg-1 compared to all reported hydrous rocking chair ZIBs. In addition, a long cycle of 6000 cycles has been obtained, and the capacity retention rate is as high as 92.1%, which is impressive. This work is expected to provide new power for the V-shaped material of the rocking chair ZIBs.
Second, guided reading of picture and text.
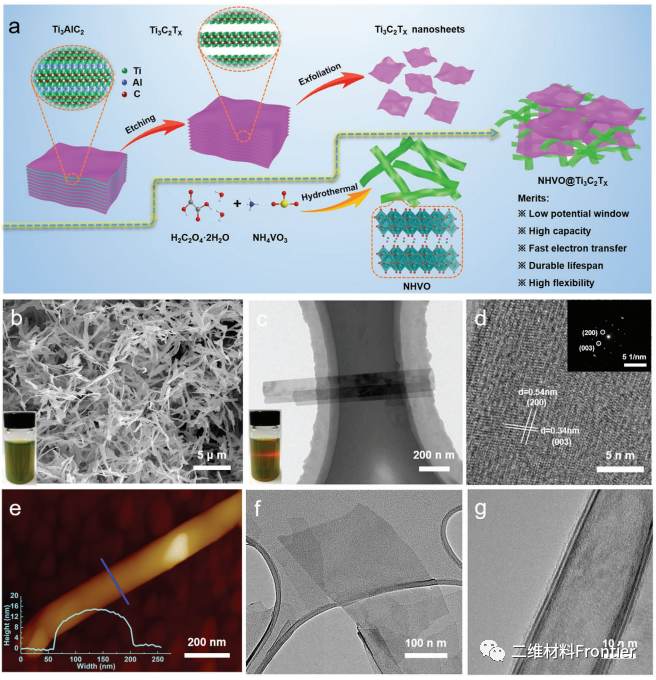
Figure 1.
A) schematic diagram of the preparation process of NHVO@Ti3C2Tx electrode. B) SEM, c) TEM, d) HRTEM (SAED of NHVO shown in the illustration). E) AFM images of NHVO nanoribbons. (the illustration shows along e. F. G. The corresponding thickness profile of the line) the TEM image of layered Ti3C2Tx nanowires.
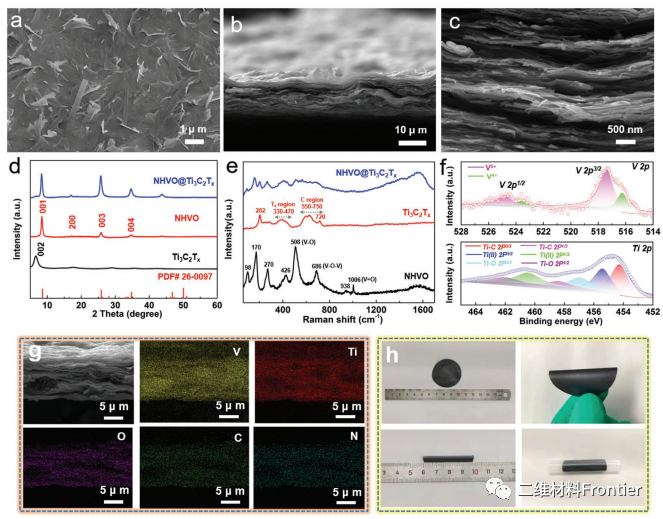
Figure 2.
A) SEM image of the surface of NHVO@Ti3C2Tx mixed film. B, c) cross-sectional SEM images of NHVO@Ti3C2Tx mixed films. D) X-ray diffraction pattern. E) Raman spectra. The NHVO spectra show the expected signals, including the stretching vibrational peaks of vanadium bonds VO (938 and 1006 cm- 1), VO V (686 cm- 1) and VO (508 cm 1). Ti3C2Tx spectra, about 330g (Ti, O, C) and A1g (C) in Tx region and C region, respectively, represent tissue surface vibration and carbon vibration, respectively, and the two distinct infection peaks of about 202and 720cm-1 are A1g (titanium, O, C) and A1g (C), respectively, and the two distinct infection peaks are about 330g (titanium, O, C) and A1g (C), respectively. F) V 2p and Ti 2p XPS spectra of NHVO@Ti3C2Tx hybrid films. G) element mapping of NHVO@Ti3C2Tx. H) Optical flexibility image of NHVO@Ti3C2Tx.
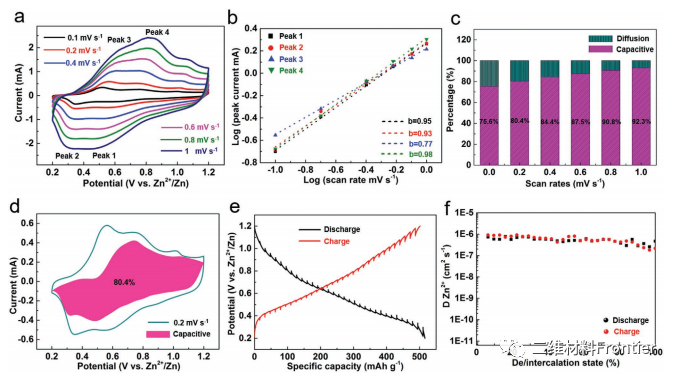
Figure 3.
Electrochemical kinetic analysis of NHVO@Ti3C2Tx electrode. A) various CV curves of NHVO@Ti3C2Tx. B) logarithmic (I) and logarithmic (v) graphs of each redox peak. C) contribution ratio of capacitance control and diffusion control. D) the CV profile shows that the contribution of capacitance to the total current (pink region) is at 0.2 mV s 1. E) GITT bends at 0.1A. F) Zn2+ diffusion coefficient (DZn).
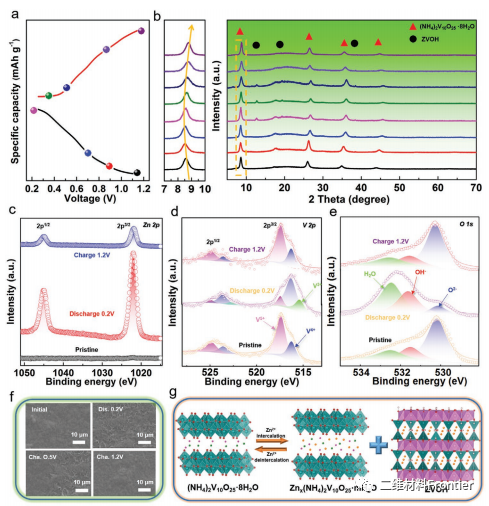
Figure 4.
Study on the reaction mechanism of NHVO@Ti3C2Tx electrode. A) the GCD profile of the third cycle at 0.1a g 1 and b) the non-in-situ XRD spectra in the selected state. C e) High resolution XPS spectra of Zn 2p, V 2p and O 1s. F) SEM images of the morphological development of NHVO@Ti3C2Tx electrodes. G) the storage mechanism diagram of Zn2+ ions.
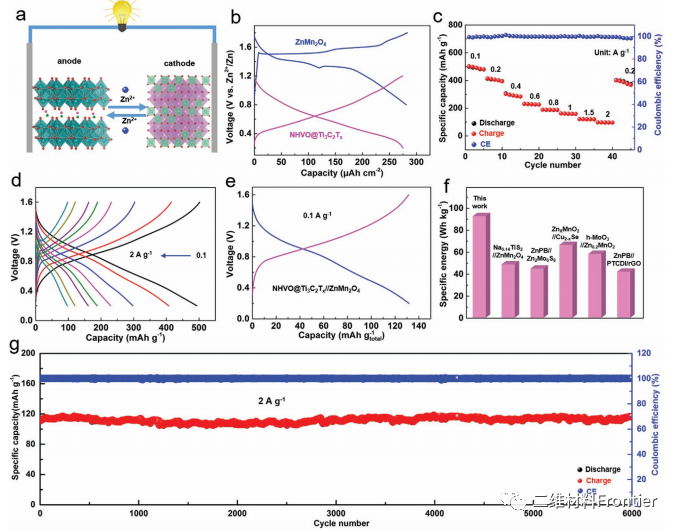
Figure 5.
Electrochemical performance of NHVO@Ti3C2Tx//ZnMn2O4 whole battery. A) NHVO@Ti3C2Tx//ZnMn2O4 full electric diagram. B) GCD curves of NHVO@Ti3C2Tx anode and ZnMn2O4 cathode at 0.1A g 1. C) the performance of the rate. D) GCD curve of NHVO@Ti3C2Tx//ZnMn2O4 battery (based on anode quality). E) the GCD curve of NHVO@Ti3C2Tx//ZnMn2O4 battery (based on the mass of anode and cathode) at 0.1A g 1. F) some reported energy density comparisons of rocking chair zinc ion full batteries. G) cycle performance of NHVO@Ti3C2Tx//ZnMn2O4 full battery at 2.0A g.
III. Summary of the full text.
To sum up, a new type of NHVO@Ti3C2Tx hybrid thin film material was synthesized for the first time and studied. The prepared NHVO nanoribbons have good dispersion and ultra-thin morphology, which is only about 14 nm. When combined with 2D Ti3C2Tx nanowires, a flexible NHVO@Ti3C2Tx mixed film electrode was obtained and showed fast ion and electron transport. Due to the crystal structure of NHVO and the assistance of Ti3C2Tx nanowires, the NHVO@Ti3C2Tx mixed film electrode can provide a high capacity of up to 514.7 mAh g 1, and more importantly, it can be cycled 6000 times at 5.0A g 1, with a retention rate of 84.2%. In addition, we verified the high performance rocking chair zinc ion full battery by pairing NHVO@Ti3C2Tx as anode and ZnMn2O4 as cathode, which provides a maximum specific capacity of 131.7 mAh Gmurl (based on total mass) and a maximum energy density of 97.1 Wh kg-1 compared to all reported waterborne rocking chair zinc ion full batteries. It is worth noting that after 6000 cycles at 2.0A g 1, the fully charged battery showed a high capacity retention of 92.1%. Therefore, this work will provide new insights for the exploration of high-density zinc non-metal anode materials for water-based zinc in energy storage applications.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.
+86-18915413828(WhatsApp&WeChat)
Previous: University of Maryland


