Nature communications | CeO2 nanoparticles with adjustable catalytic activity can prevent chemotherapy-induced acute kidney injury
Introduction
Acute kidney injury (AKI) is a common and fatal side effect caused by tumor chemotherapy, which seriously affects cancer patients receiving chemotherapy. AKI is mainly manifested by ROS induced renal cell injury. Reducing ROS can reduce AKI caused by chemotherapy, and at the same time, it can also reduce the effect of chemotherapy. Small molecule antioxidant N-acetylcysteine (NAC) is a way to reduce oxidative damage and resist chemotherapy-induced AKI; As a kind of nano antioxidant, Mo based polyoxometalate nanoclusters can aggregate in kidney to protect kidney effectively; DNA origami nanostructures have also been confirmed to have ROS scavenging ability to protect kidney structure and improve AKI. However, the antioxidant effect of these small molecules and nano materials will also reduce the oxidative stress of tumor tissue, stimulate the growth and metastasis of tumor, thus hindering their application in chemotherapy-induced AKI.
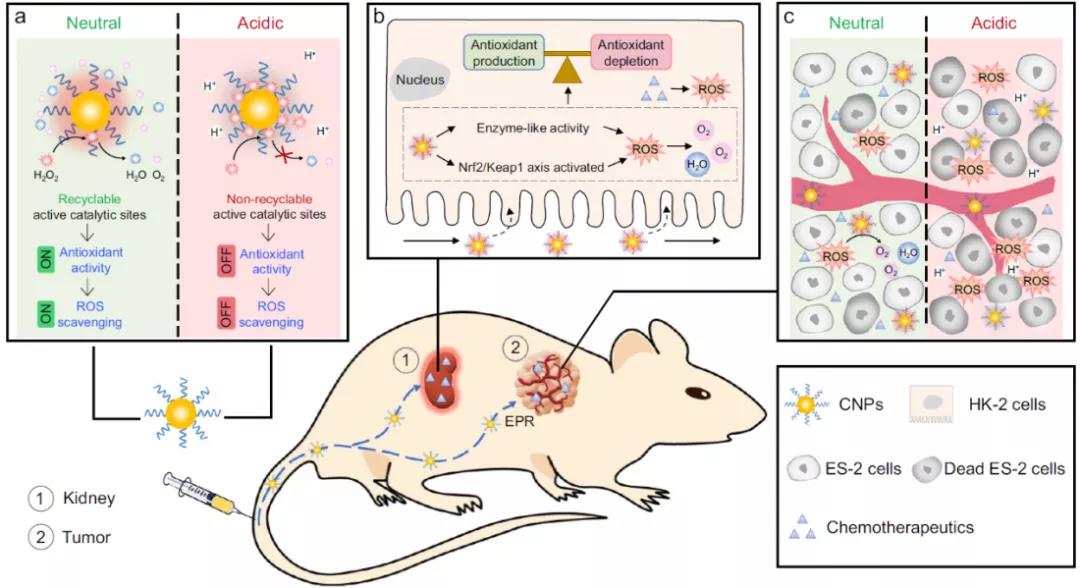
In view of this, Ling daishun research group of Zhejiang University published the research on catalytic activity tunable ceria nanoparticles prevent chemistry induced acute kidney injury without interference with chemistry in nature communication, It can prevent AKI caused by chemotherapy, but it will not interfere with the effect of chemotherapy. Specifically, in the neutral environment of renal cortex, CNP catalyzes the decomposition of H2O2, and then regulates the expression of ROS related genes by activating Nrf2 / Keap1 signaling pathway to restore the balance of ROS and protect renal tubules; In the acidic tumor microenvironment, CNP does not interfere with ROS generated by chemotherapy to kill cancer cells because high concentration of H + destroys its active catalytic sites and limits its antioxidant activity. As a ROS regulator, CNP has great potential in the clinical prevention and treatment of AKI in cancer patients.
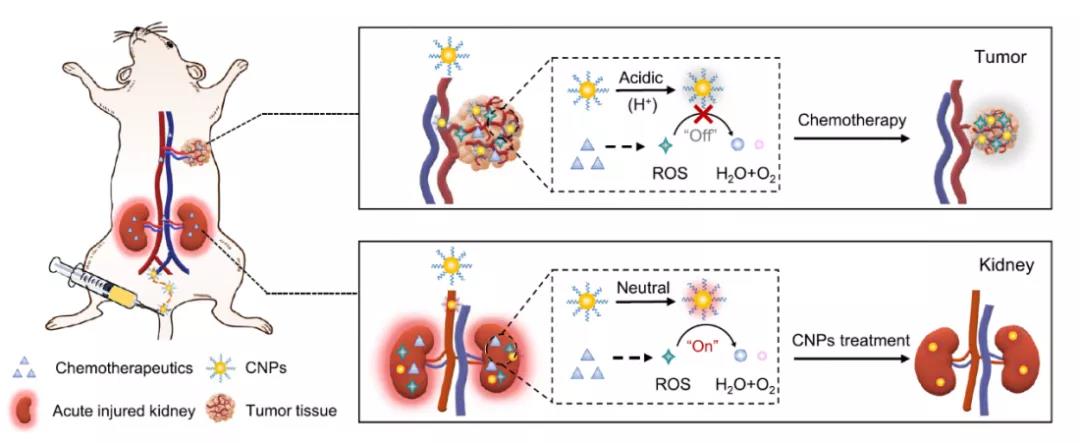
Fig. 1 Schematic diagram of CNPs showing different functions in different tissues of small animals due to different pH.
research contents
01
Synthesis and characterization of CNPs
Hydrophobic CNPs with a diameter of about 3 nm were synthesized by an improved reverse micelle method, and their performance was modified by dspe-peg2k. The hydration kinetic diameter of CNPs is 9.7 nm and the potential is - 20.4 MV. XPS shows that Ce3 + and Ce4 + coexist. The degradation of H2O2 induced by CNPs was in accordance with Michaelis Menten kinetic equation. Km is 1.4 times lower and Vmax is 2.1 times higher in neutral condition than in acidic condition. XRD results show that the tunable activity of CNPs is not based on the change of crystal structure. H2O2 + 2ce4 + → O2 + 2H + + 2ce3 + + VO, Ce3 + can return to Ce4 + in acidic environment and re form active site, but not activated CNP (ICNP). The antioxidant activity of CNPs permeated by H2O2 and H + loses and is difficult to reverse.
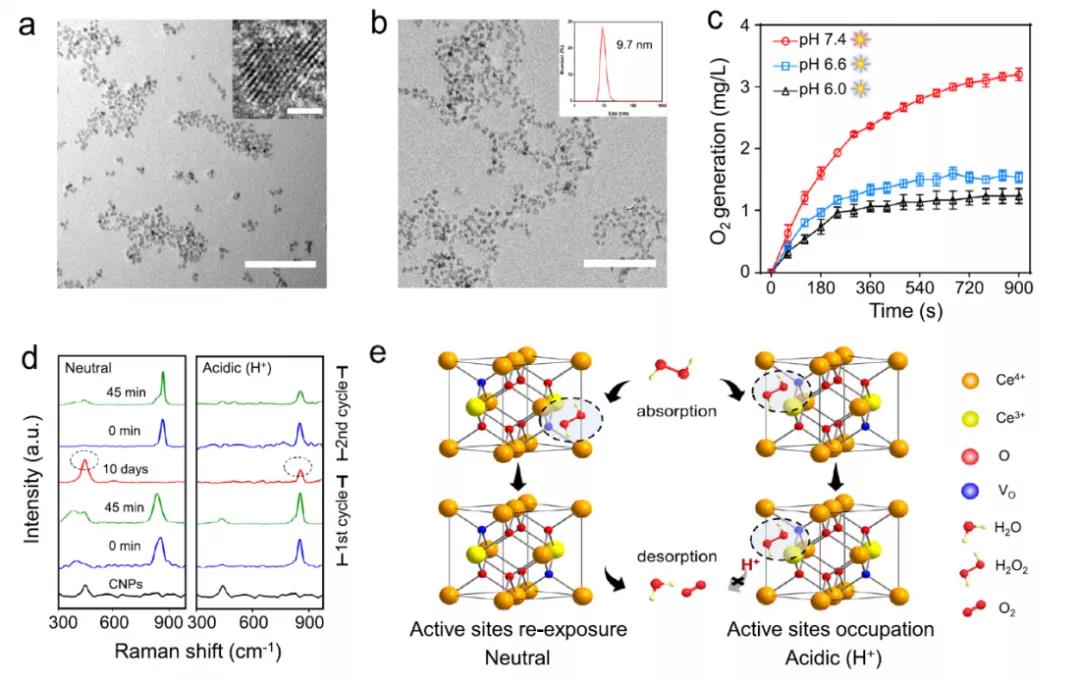
Fig. 1 (a) TEM images of ultramicro CNPs in chloroform( b) In the TEM images of DSPE-PEG modified CNPs in aqueous phase, DLS data showed that the diameter distribution of DSPE-PEG was 9.7 nm( c) The amount of O2 produced by the decomposition of H2O2 by CNPs at different pH values( d) At neutral and acidic pH, CNPs reacted with H2O2 without time( e) The chemical model diagram of catalase like activity difference in pH environment dependence of CNPs.
02
Condition dependent cytoprotective effect of CNPs
Cisplatin (DDP) is a widely used anti-tumor drug with nephrotoxicity. The reason is that renal epithelial cells are the main target of DDP induced nephrotoxicity. HK-2 cells and ES-2 cells were used to study the pH dependent protective effect of CNPs on kidney. The results showed that CNP significantly reduced DDP induced cytotoxicity at pH 7.4, but not at pH 6.6 or pH 6.0; However, NAC, a small molecule antidote, still has anti toxicity at pH = 6.6. In other tumor cells and normal cells, such as ovcar8, HepG2, A549 and normal LO2 cells, CNPs showed pH dependent cytoprotective effect against DDP. In addition, CNPs have pH dependent anti taxol cytotoxicity in HK-2 and ES-2 cells.
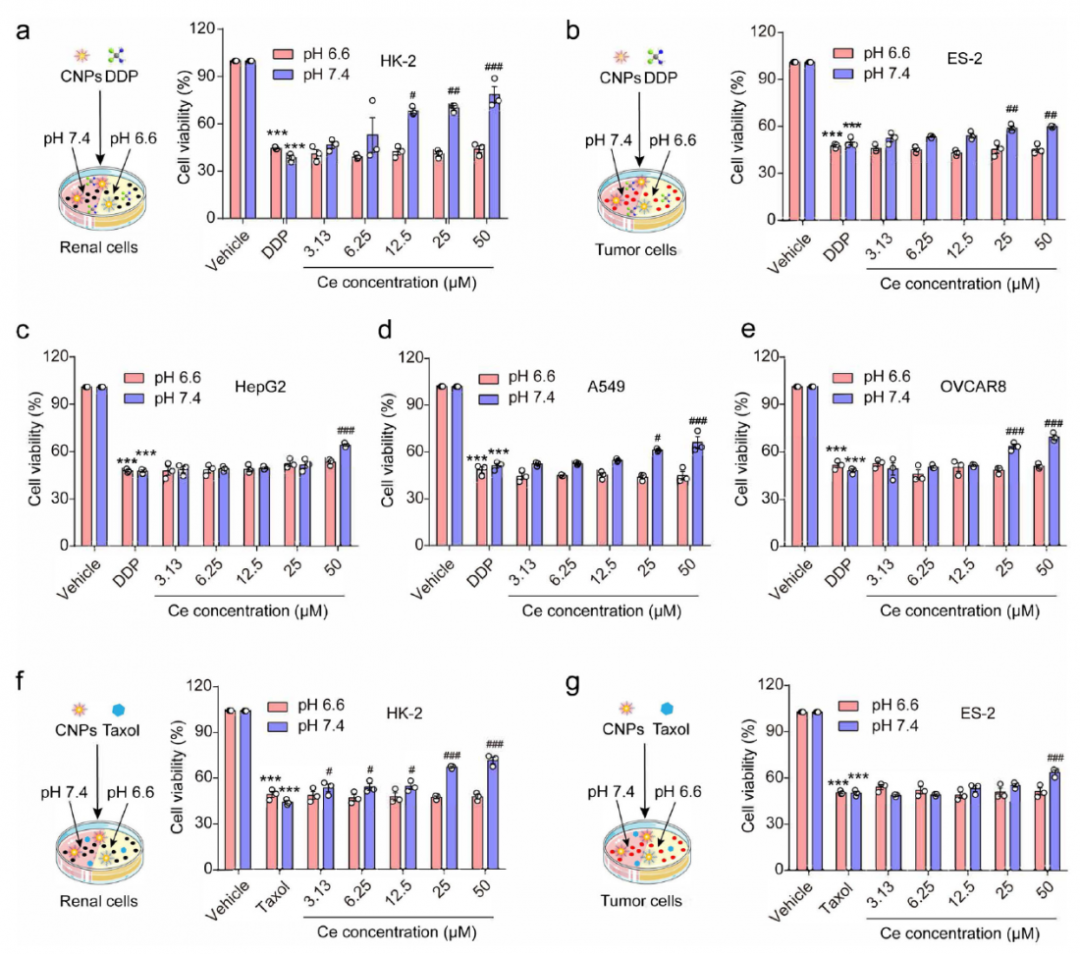
Fig. 2 (A-E) the effects of different concentrations of CNPs on the survival rates of HK-2, ES-2, HepG2, A549 and ovcar8 under different pH conditions( f. G) the survival rate of HK-2 and ES-2 cells treated with paclitaxel under different pH conditions.
03
Effect of CNPs on chemotherapy-induced AKI in vivo
The researchers further studied the pharmacokinetics and distribution of CNPs in mice. Histology showed that CNPs had no obvious cytotoxicity. Compared with normal mice, the accumulation of CNPs in AKI mice was increased and the retention time in renal epithelium was longer. Biological electron microscopy showed that there were CNPs in the cilia of renal tubular epithelial cells, glomerular basement membrane and renal tubules. This may be related to the small size of CNPs (~ 3nm), the deformable surface modification of PEG, the negative surface charge of CNPs and the enhancement of anti glomerular basement membrane (GBM) permeability. CNPs were found in the urine of treated mice, indicating that CNPs could be filtered into renal tubules through GBM and excreted through urine. Caveolae mediated endocytosis plays an important role in the uptake of CNPs. The levels of serum urea nitrogen (BUN), creatine (CRE) and KIM-1 mRNA were decreased in AKI mice after injection of CNPs. H & E staining showed that CNPs could improve the renal injury induced by DDP.
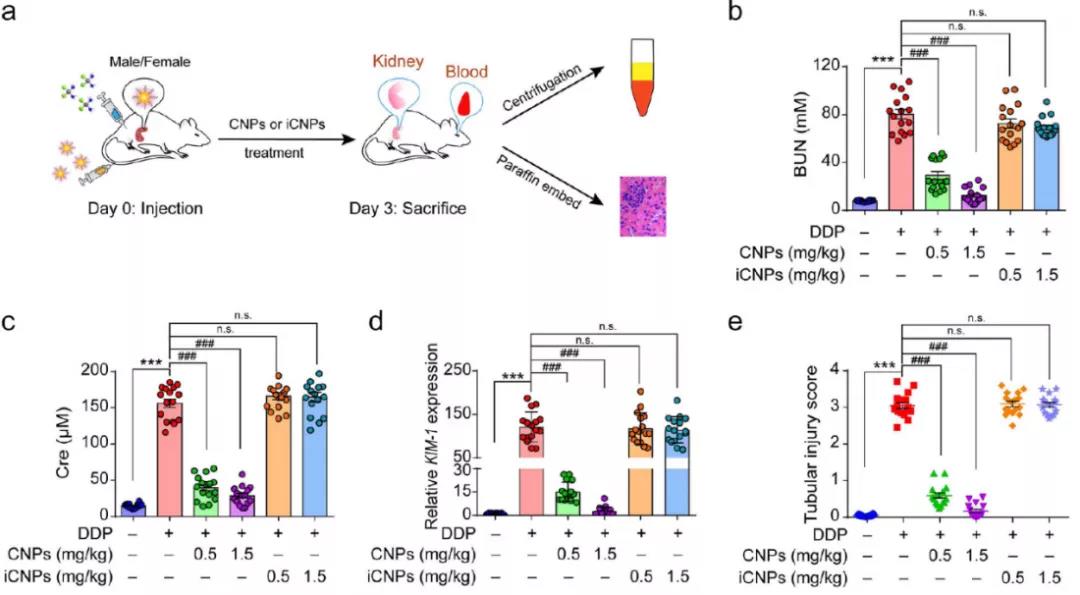
Fig. 3 (a) schematic diagram of treatment and evaluation of AKI mice( b. C and D) the expression of bun, CRE and KIM-1 related genes after different treatments( e) The degree of renal tubular injury after different treatments was analyzed.
04
Anti apoptotic effect of CNPs in AKI
The researchers explored the mechanism of CNPs reducing AKI through the effect of CNPs on apoptosis signal. After DDP treatment, the apoptosis rate and the expression of PARP and Caspase-3 in HK-2 cells were significantly increased, but those related factors were significantly decreased after CNPs treatment. Therefore, CNPs can significantly reduce the level of TUNEL apoptosis induced by DDP. ROS is the key factor in inducing apoptosis. CNPs can clear the endogenous ROS induced by DDP, reverse the inhibition of SOD and the increase of MDA induced by DDP. QRT PCR analysis showed that CNPs could reduce HO-1 gene expression and increase NOX2 gene expression after DDP treatment, making their expression close to the original level without DDP treatment.
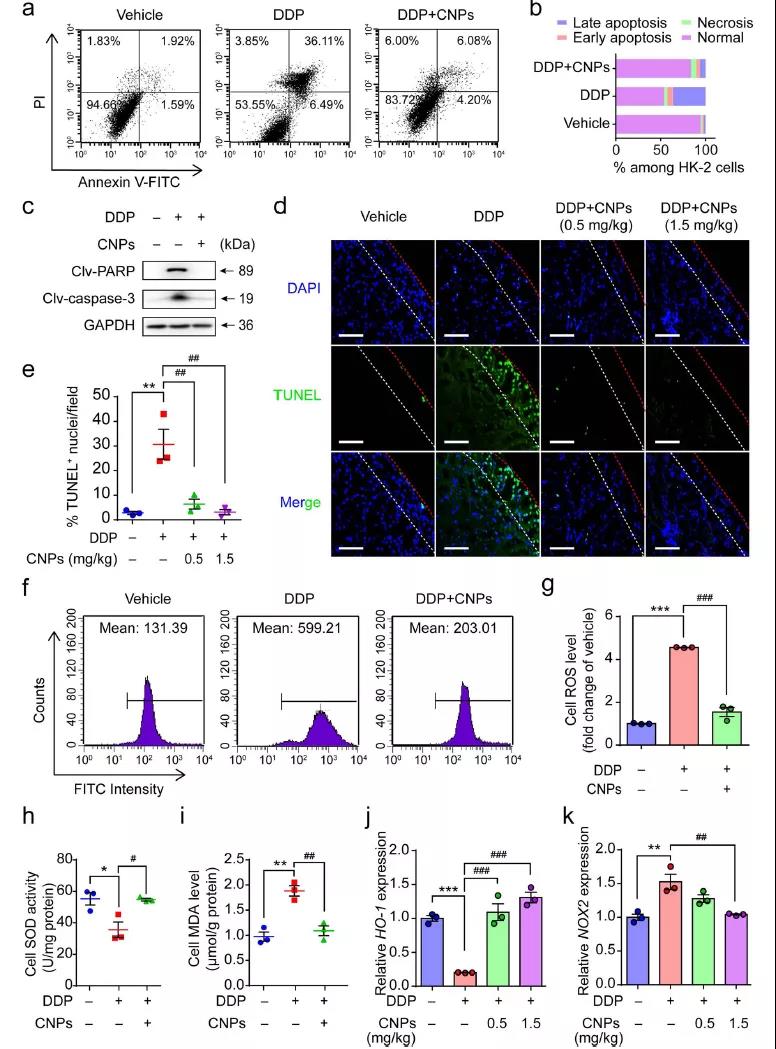
Fig. 4 (a) flow cytometry analysis of apoptosis( b) HK-2 cells in various states were quantified( c) The expression of PARP and caspase-3 was analyzed by Western blot( d. E) staining chart and chart of TUNEL level( f. G) flow cytometric analysis of ROS in HK-2 cells( H-K) SOD activity, MDA level, HO-1 and NOX2 related gene expression.
05
Molecular mechanism of protective effect of CNPs on Aki
Nrf2 is the main regulator in ROS signaling pathway (the expression of Nrf2 decreases with the increase of ROS). Western bolt was used to detect the expression of Nrf2 and its related proteins DJ-1 and Keap1 before and after CNPs treatment. It was found that the expression of DJ-1 and Nrf2 was up-regulated, while the expression of Keap1 was down regulated. Nrf2 knockout can eliminate the protective effect of CNPs on AKI induced by DDP, and can not inhibit the expression levels of apoptosis related PARP and caspase-3. Antioxidant genes are no longer sensitive to CNPs. These results indicate that CNPs can protect renal cells by activating Nrf2 to reduce ROS and thus prevent apoptosis.
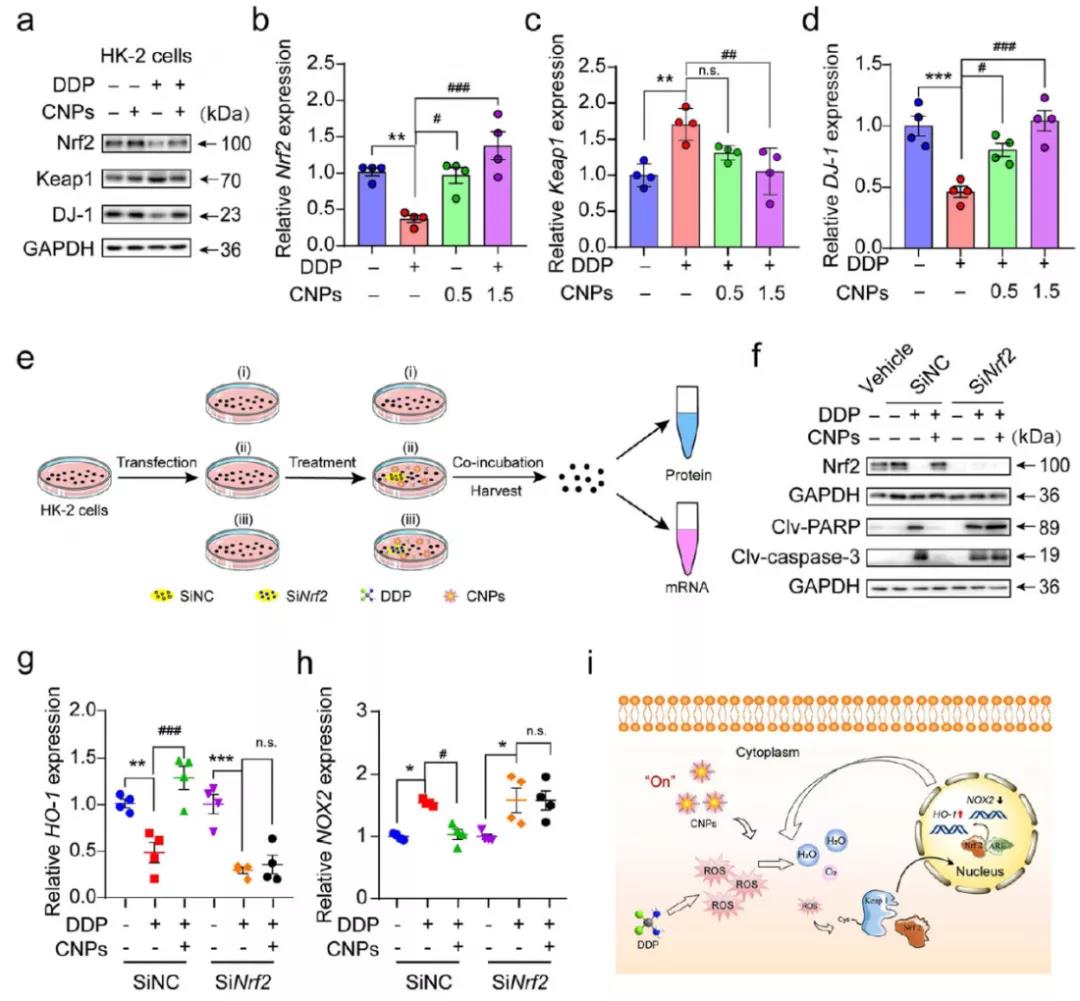
Fig. 5 (a, B-D) Western blot analysis and quantification of Nrf2, Keap1 and DJ-1 in HK-2 cells after different treatments( Western blot and QRT PCR were used to analyze the expression of Nrf2( i) The intracellular signal transduction of CNPs against DDP induced oxidative stress by activating Nrf2 / Keap1.
06
Effect of CNPs on overall chemotherapy in vivo
Compared with small molecule drug NAC (which also increases SOD activity and reduces MDA level in tumor), CNPs (which does not change SOD activity and does not reduce MDA level in tumor) can enhance the therapeutic effect of chemotherapy drugs on tumor to a greater extent and reduce the side effects of chemotherapy.
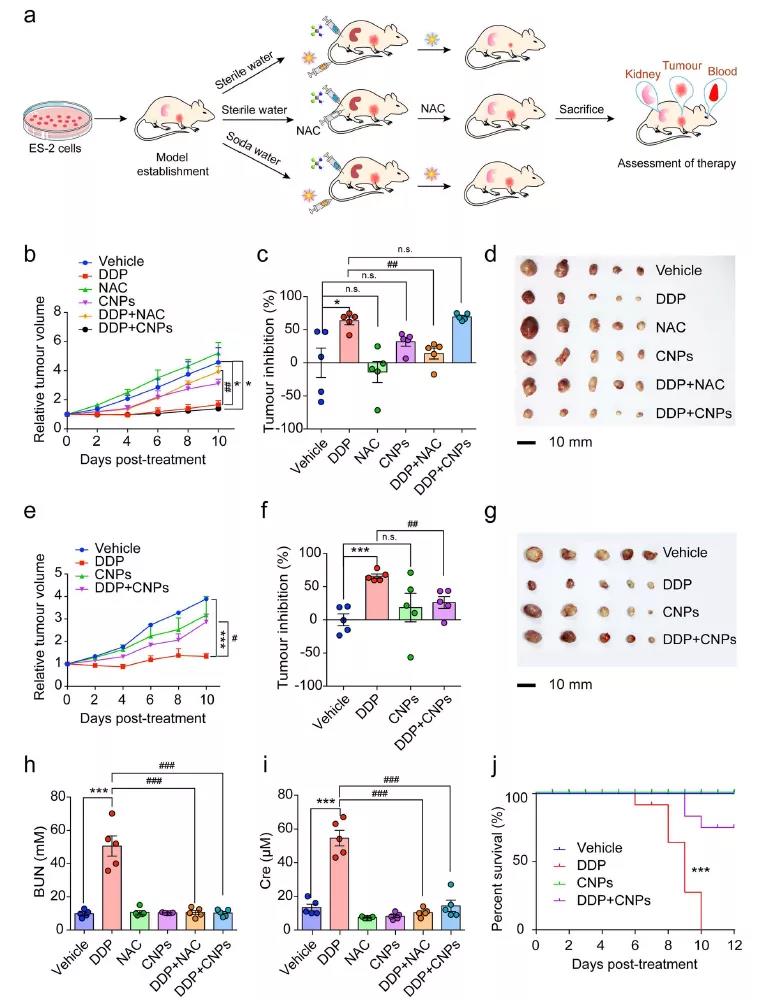
Fig. 6 (a) treatment and evaluation of ES-2 subcutaneous tumor mice( B-G) tumor volume, inhibition efficiency and solid tumor size of different treatments( h. I) the levels of bun and CRE in mice under different treatments and (J) the survival rate of mice under different treatments.
Summary and Prospect
In conclusion, AKI is a high mortality disease associated with ROS and a common side effect of chemotherapy. Small molecule antioxidant (NAC) can reduce the effect of chemotherapy while reducing AKI. Based on the pH dependent activity adjustable CNPs, in the normal pH neutral cell environment, they can decompose H2O2 adsorbed on the surface, re expose the active catalytic sites of CNP for the next cycle, thus reducing the ROS in cells, reducing the apoptosis of cells, and reducing the toxic and side effects of chemotherapy drugs; However, in the pH acidic environment of tumor cells, the H2O2 adsorbed on the surface of tumor cells will destroy the re exposure of active catalytic sites and prevent the ROS clearance, without reducing the tumor killing ability of chemotherapeutic drugs. CNPs protect pH neutral cells by activating Nrf2 / Keap1 signaling pathway, increasing SOD activity, increasing the expression of antioxidant genes, regulating cell apoptosis and related protein expression, so as to reduce ROS, apoptosis and renal injury. Therefore, CNPs are expected to expand the use of chemotherapy, so that more cancer patients can benefit from it.
This information is from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately
+86-18915413828(WhatsApp&WeChat)
Previous: Wuhan virus Institute
Next: Biological microsphere


