Academician Chen Xuesi of Changchun Institute of Applied Chemistry and Associate Researcher Xiao Chunsheng "AFM": Development of tandem dynamic covalent bond, injectable, self-healing hydrogel wound d
For a long time, hydrogel can maintain a moist environment at the wound interface, absorb wound exudate, act as a microbial barrier and promote wound healing. Injectable hydrogels formed in situ under physiological conditions are considered to be the most promising wound healing materials. ] However, most injectable hydrogels experience a relatively long gel time, which causes the polymer to diffuse from the target site and induce wound infection. In addition to covering the wound quickly, it is also important to change the dressing under mild conditions. Because routine cutting or mechanical debridement during dressing changes may lead to peeling of the new epidermis, resulting in wound enlargement and delayed healing. Therefore, more effective cross-linking methods are needed to develop injectable hydrogels with rapid gelation and dissolution rate.

Academician Chen Xuesi and associate researcher Xiao Chunsheng of Changchun Institute of Applied Chemistry developed tandem dynamic covalent bonds based on 2-formylphenylboronic acid (2-FPBA), including the dynamics of uncatalyzed Knoevenagel condensation (CKC) reaction and formation of borate esters. The C=C double bond is used to construct an on-demand injectable hydrogel (process 1A). The hydrogel was prepared by mixing 2-FPBA with cyanoacetate end-functionalized 4-arm PEG (4-arm PEG-CA) and polyvinyl alcohol (PVA). Due to the tandem dynamic covalent bond, the resulting hydrogel is injectable. In the presence of cysteine, the hydrogel rapidly dissolves by forming a thiazolidine borate (TzB) complex (Scheme 1B). This hydrogel can be used as a dressing for wound treatment, and achieves rapid wound closure and on-demand dissolution (Scheme 1C). The article "Injectable Self-Healing Hydrogel Wound Dressing with Cysteine-Specific On-Demand Dissolution Property Based on Tandem Dynamic Covalent Bonds" was published on "Advanced Functional Materials".
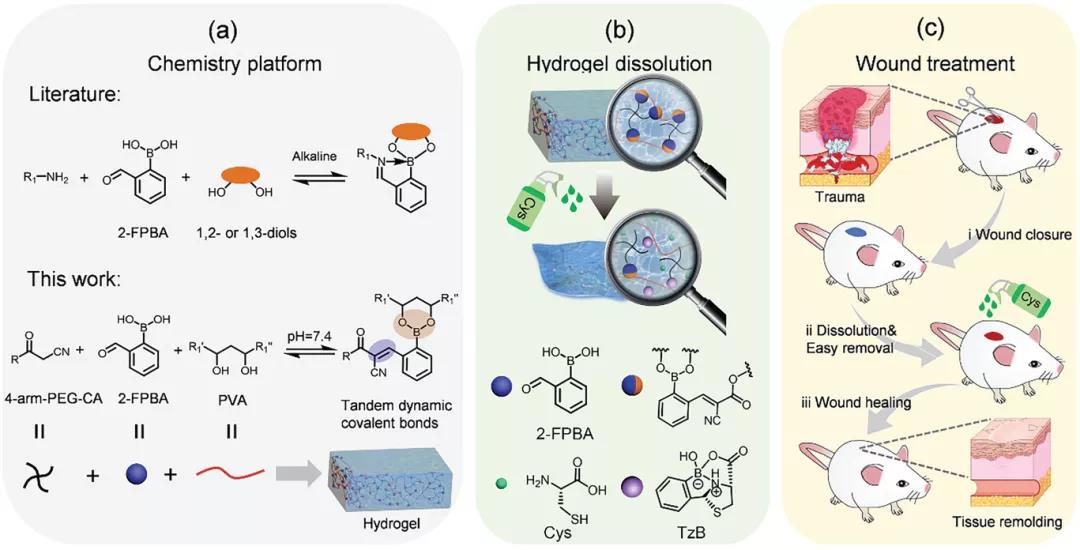
Process 1, a schematic diagram of the preparation of injectable self-healing hydrogel. A) The hydrogel is prepared by the reaction of 2-FPBA with PVA and 4-arm PEG-CA. B) When exposed to PBS cysteine solution, the 2-FBC hydrogel dissolves. C) When used as a dressing, 2-FBC hydrogel can quickly close the wound and dissolve it as needed by cysteine.
Results and discussion
【Synthesis and dissolution mechanism of 2-FBC hydrogel】
The author first measured the UV-vis spectrum to confirm the proposed mechanism of the reaction between cysteine and 2-FPBA. The new peak at 300 nm was attributed to the formation of the C=C double bond, indicating the reaction kinetics of the CKC reaction; Cystine greatly reduces the absorbance values at 300 and 254 nm, indicating that cysteine can quickly decompose mPEG-CA-BA (Figure 1C). 1H NMR confirmed that mPEG-CA reacted with 2-FPBA to form new conjugates mPEG-CA-BA and the dissociation of mPEG-CA-BA (Figure 1D). In addition, the coexistence of the reactant (mPEG-CA and 2-FPBA) and the product (mPEG-CA-BA) indicates that the C=C double bond is a dynamic covalent bond. ESI-MS and FTIR spectra confirmed the formation of TzB complexes (Figure 1E-F). These results indicate that the dynamic C=C double bond formed by the conjugation of mPEG-CA and 2-FPBA can be specifically dissociated by cysteine.
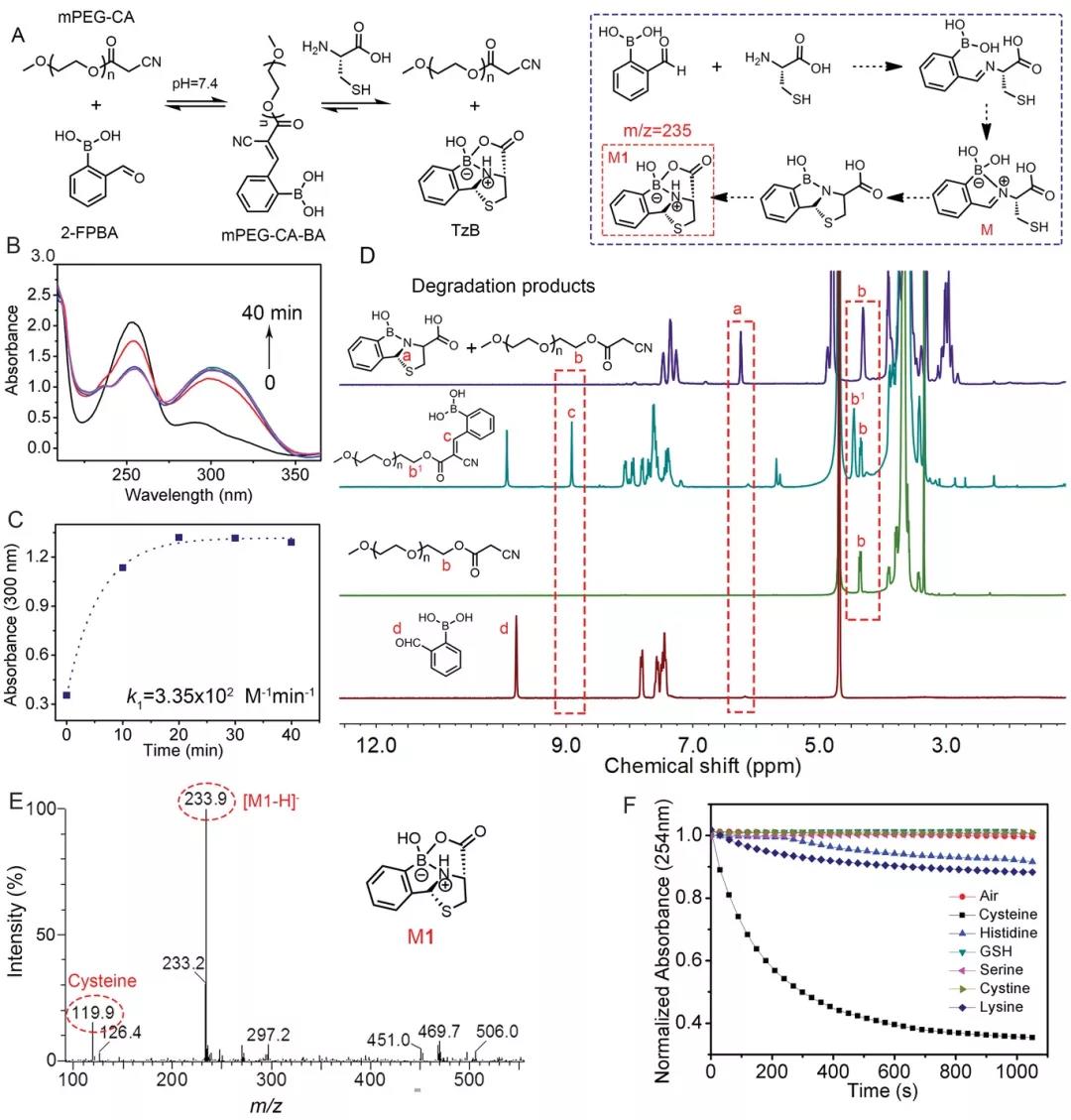
Figure 1, A) 2-FPBA reacts with mPEG-CA to form mPEG-CA-BA, and cysteine induces the dissociation of mPEG-CA-BA. B, C) UV-vis absorption spectrum when mPEG-CA and 2-FPBA react at 37°C. D) 1 H NMR spectrum of 2-FPBA, mPEG-CA, a mixture of 2-FPBA and mPEG-CA and a mixture of 2-FPBA and mPEG-CA after 10 minutes of cysteine treatment. E) Cysteine treatment ESI-MS spectrum of the mixture of 2-FPBA and mPEG-CA after 10 minutes. F) In the presence of designated small molecules, the monomer disappearance graph of (2-FPBA) in the reaction mixture.
[2-FBC hydrogel’s gelation, injectability, and self-healing ability]
Next, the author prepared 2-FBC hydrogel by mixing 4-arm PEG-CA polymer, 2-FPBA and PVA aqueous solution in a PBS solution at pH 7.4 (Figure 2A). The hydrogel is formed within 10 s, and its storage modulus increases with the increase of polymer concentration (Figure 2B). The hydrogel exhibits shear thinning and self-healing properties. When the shear stress exceeds 1000 Pa, G'is lower than G'', indicating that the 2-FBC hydrogel network ruptures under high stress (Figure 2C). When the shear rate was increased from 0.1 to 100 s-1, the viscosity of the hydrogel dropped sharply, indicating good shear thinning characteristics (Figure 2D-2E). When the hydrogel is strained at 500%, G'immediately drops to 0 Pa, and the hydrogel network is destroyed; when the strain turns to 1%, its modulus is completely restored within a few seconds (Figure 2F). The hydrogel can be lifted and bear a load of 3.37 g (Figure 2G). These results confirm that 2-FBC hydrogel has rapid gelation, excellent injectability and effective self-healing ability, which is conducive to wound healing.
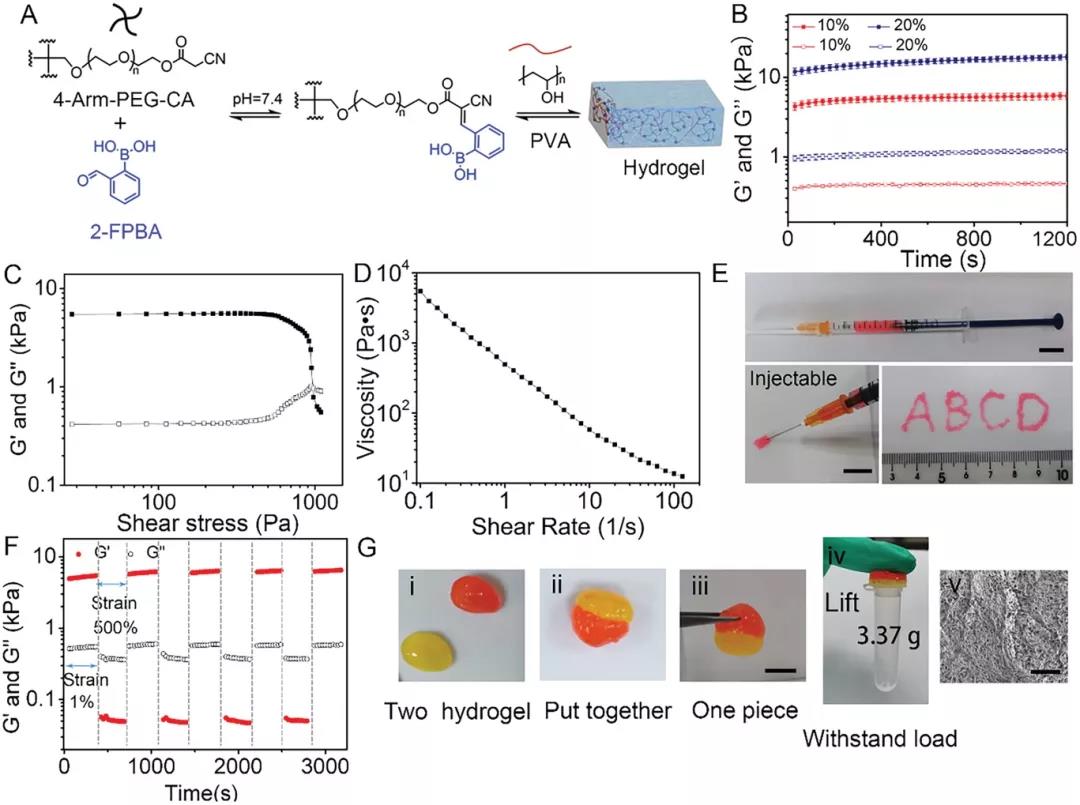
Figure 2. Preparation and characterization of injectable self-healing hydrogels by 2-FPBA-mediated three-component condensation. A) Hydrogel formed by 4-arm PEG-CA, PVA and 2-FPBA. B) Storage modulus (Gʹ) and loss modulus (Gʺ) of 2-FBC hydrogel at different concentrations at 37°C. C) Shear of 10% 2-FBC hydrogel at 37°C Shear stress scanning test. D) Viscosity of 10% 2-FBC hydrogel. E) 10% 2-FBC hydrogel injectability. F) Evaluate 10% at room temperature with alternating strains of 1% and 500% 2 -Self-healing ability of FBC hydrogel. G) 2-FBC hydrogel self-healing process.
[Cysteine induces dissolution of 2-FBC hydrogel]
After 20 minutes of cysteine treatment, the 2-FBC hydrogel was completely dissolved (Figure 3B). Increasing the concentration of cysteine from 0 to 0.2 M resulted in an increase in the degradation rate and a decrease in the dissolution time from 1440 to 20 minutes. If adding glucose can accelerate the degradation, this may be due to the dissociation of the borate bond in the tandem dynamic covalent bond induced by glucose. The SEM image showed that after the solution, the pores of the hydrogel became smaller and more irregular, indicating that the hydrogel network was destroyed. Next, the author studied the on-demand dissolution characteristics of 2-FBC hydrogel on the skin surface (Figure 3H). The 2-FBC hydrogel was injected into pig skin, and then half of the hydrogel was covered with gauze soaked in cysteine. After 20 minutes, the part covered by the cysteine-soaked gauze was completely dissolved.
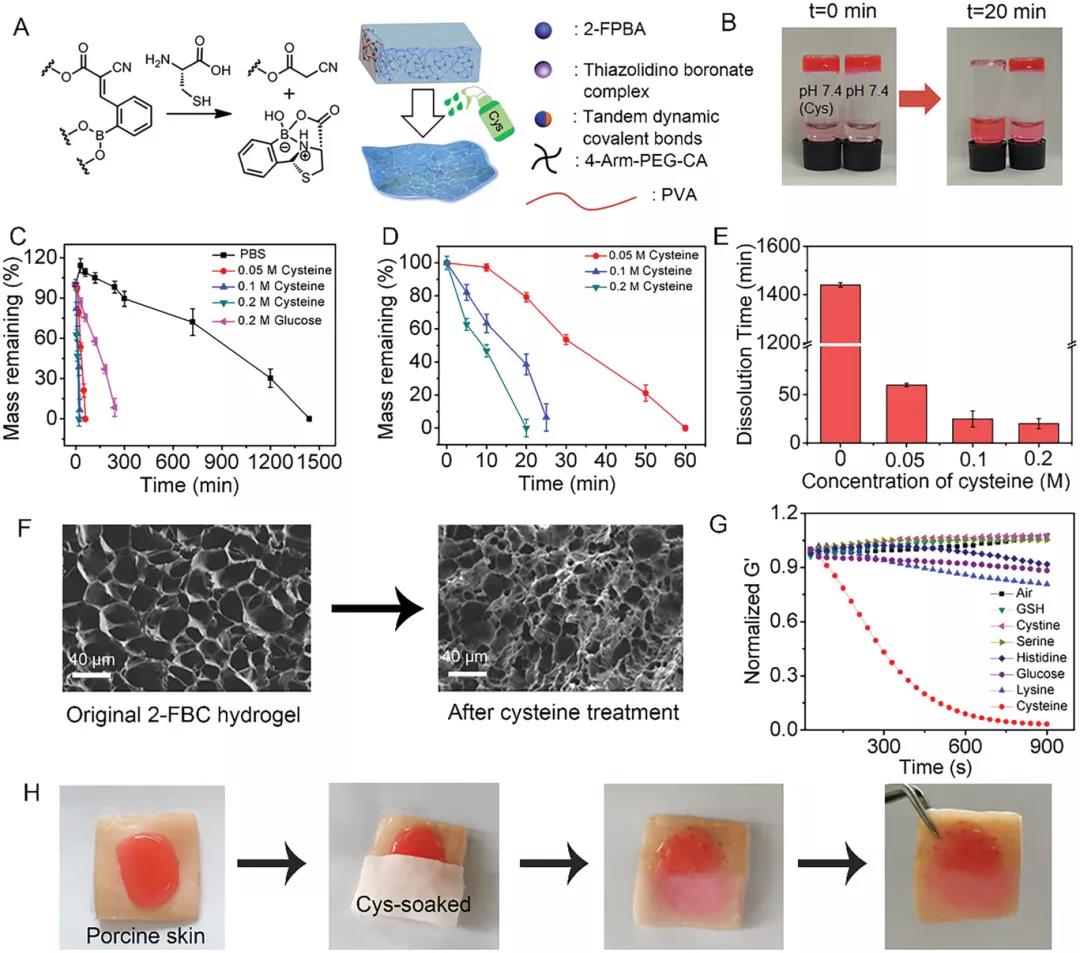
Figure 3. On-demand dissolution of 2-FBC hydrogel. A) Schematic diagram of 2-FBC hydrogel dissolution. B) Images of 2-FBC hydrogel before and after treatment with PBS (pH 7.4) or Cys solution (0.2 M, pH 7.4) for 20 minutes. C, D) Degradation of 2-FBC hydrogel in PBS (pH 7.4), PBS (pH 7.4) + 0.2 M glucose and PBS (pH 7.4) + cysteine. E) The dissolution time of 2-FBC hydrogel in PBS (pH 7.4) containing different concentrations of cysteine. F) SEM images of 2-FBC hydrogel before and after treatment with cysteine (0.2 m) for 10 minutes. G) Modulus changes of solutions (pH 7.4) exposed to biomolecules (glutathione, cystine, serine, histidine, glucose, lysine and cysteine). H) 2-FBC hydrogel adheres to pig skin tissue and is dissolved in cysteine soaked gauze as needed.
【2-FBC hydrogel promotes wound healing】
Next, the rat skin full-thickness defect model was used to evaluate the wound healing effect of 2‐FBC hydrogel. After 7 days of treatment, the wound closure rate of hydrogel treatment reached 73.5%. On the 15th day, no obvious wound was observed in the hydrogel treatment group. The wound healing effect of cysteine treatment is more attributable to cysteine's ability to reduce inflammation during dressing changes. In addition, hydrogel-treated wounds show fewer inflammatory cells, which may be due to the hydrogel wound dressing's ability to isolate the wound from the external environment. On the 7th day of treatment, all hydrogel treatment groups formed an epithelial layer, and after 15 days, more hair follicles and sebaceous glands were also observed, and denser collagen fibers were displayed at the incision.
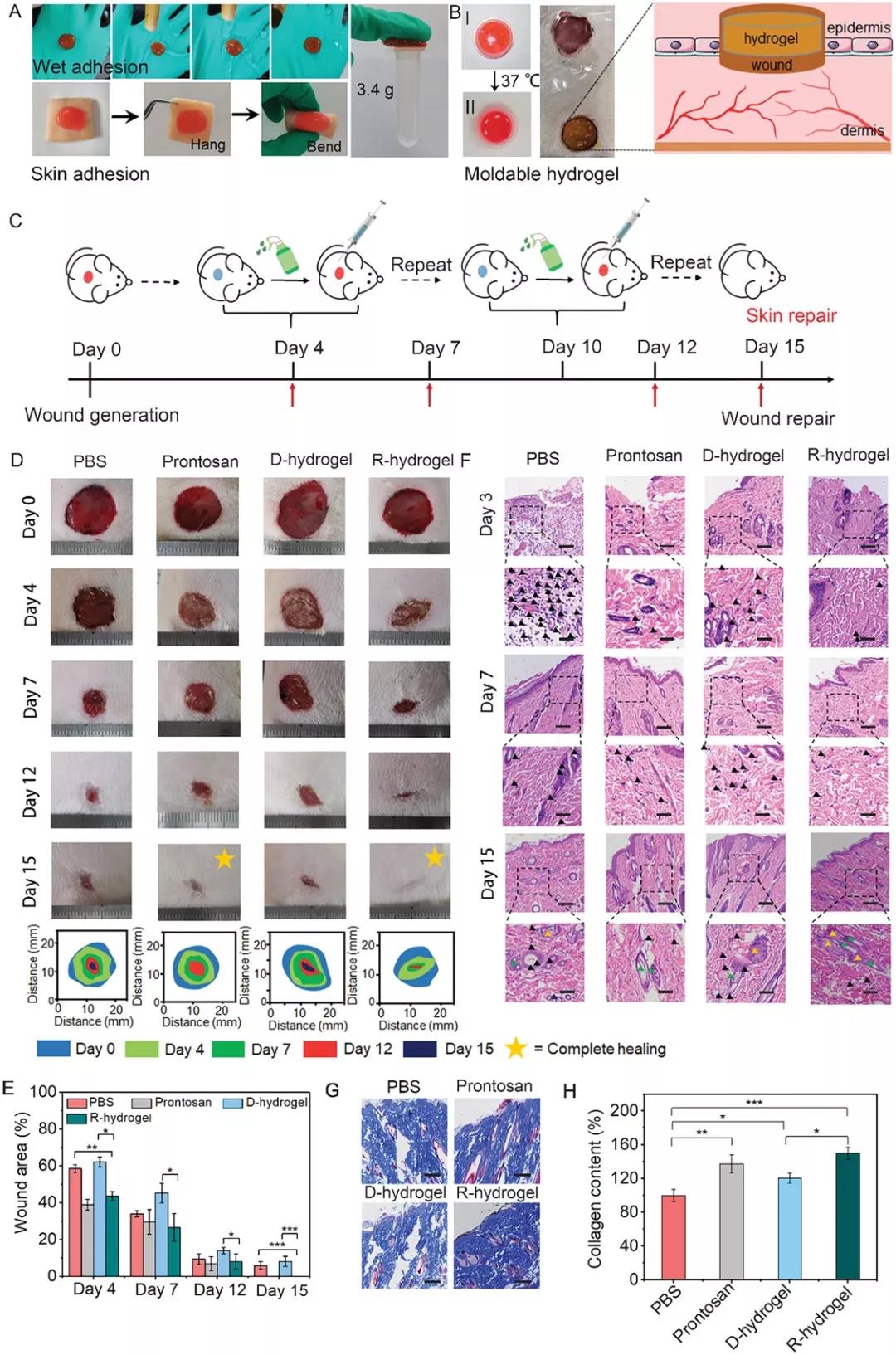
Figure 4. Adhesion behavior of 2-FBC hydrogel and wound healing effect in vivo. A) After rinsing, the 2-FBC hydrogel adheres firmly to the rubber gloves and can withstand a load of 3.4 g. B) 2-FBC hydrogel can be formed. C) Schematic diagram of 2-FBC hydrogel wound treatment process. D) Use PBS (pH 7.4), commercially available wound dressing Prontosan, D-hydrogel (inject 2-FBC hydrogel into the wound area and then perform surgical excision) and R-hydrogel (hydrocoagulate 2-FBC) Glue is injected into the wound area and removed by cysteine-induced dissolution). F) H&E stained histological images of wound tissue in each treatment group. G) Masson stained histological image of skin tissue regeneration on the 15th day. H) Collagen content in different treatment groups based on Masson staining.
Conclusion: In short, the author has developed a series of new tandem dynamic covalent bonds composed of C=C double bonds composed of CKC reaction and borate esters, which are used to construct fast gelation and specific on-demand dissolution characteristics of cysteine Hydrogel dressing. The hydrogel is injectable, self-healing, and can be specifically degraded by cysteine by forming TzB complexes. When used as a wound dressing to treat full-thickness wounds, the hydrogel can quickly close the wound within seconds, and can also be dissolved as needed to change the dressing. This dynamic hydrogel opens up new ways for wound dressings and further expands the application of dynamic hydrogels based on Knoevenagel condensation reaction.

Academician Chen Xuesi and associate researcher Xiao Chunsheng of Changchun Institute of Applied Chemistry developed tandem dynamic covalent bonds based on 2-formylphenylboronic acid (2-FPBA), including the dynamics of uncatalyzed Knoevenagel condensation (CKC) reaction and formation of borate esters. The C=C double bond is used to construct an on-demand injectable hydrogel (process 1A). The hydrogel was prepared by mixing 2-FPBA with cyanoacetate end-functionalized 4-arm PEG (4-arm PEG-CA) and polyvinyl alcohol (PVA). Due to the tandem dynamic covalent bond, the resulting hydrogel is injectable. In the presence of cysteine, the hydrogel rapidly dissolves by forming a thiazolidine borate (TzB) complex (Scheme 1B). This hydrogel can be used as a dressing for wound treatment, and achieves rapid wound closure and on-demand dissolution (Scheme 1C). The article "Injectable Self-Healing Hydrogel Wound Dressing with Cysteine-Specific On-Demand Dissolution Property Based on Tandem Dynamic Covalent Bonds" was published on "Advanced Functional Materials".

Process 1, a schematic diagram of the preparation of injectable self-healing hydrogel. A) The hydrogel is prepared by the reaction of 2-FPBA with PVA and 4-arm PEG-CA. B) When exposed to PBS cysteine solution, the 2-FBC hydrogel dissolves. C) When used as a dressing, 2-FBC hydrogel can quickly close the wound and dissolve it as needed by cysteine.
Results and discussion
【Synthesis and dissolution mechanism of 2-FBC hydrogel】
The author first measured the UV-vis spectrum to confirm the proposed mechanism of the reaction between cysteine and 2-FPBA. The new peak at 300 nm was attributed to the formation of the C=C double bond, indicating the reaction kinetics of the CKC reaction; Cystine greatly reduces the absorbance values at 300 and 254 nm, indicating that cysteine can quickly decompose mPEG-CA-BA (Figure 1C). 1H NMR confirmed that mPEG-CA reacted with 2-FPBA to form new conjugates mPEG-CA-BA and the dissociation of mPEG-CA-BA (Figure 1D). In addition, the coexistence of the reactant (mPEG-CA and 2-FPBA) and the product (mPEG-CA-BA) indicates that the C=C double bond is a dynamic covalent bond. ESI-MS and FTIR spectra confirmed the formation of TzB complexes (Figure 1E-F). These results indicate that the dynamic C=C double bond formed by the conjugation of mPEG-CA and 2-FPBA can be specifically dissociated by cysteine.

Figure 1, A) 2-FPBA reacts with mPEG-CA to form mPEG-CA-BA, and cysteine induces the dissociation of mPEG-CA-BA. B, C) UV-vis absorption spectrum when mPEG-CA and 2-FPBA react at 37°C. D) 1 H NMR spectrum of 2-FPBA, mPEG-CA, a mixture of 2-FPBA and mPEG-CA and a mixture of 2-FPBA and mPEG-CA after 10 minutes of cysteine treatment. E) Cysteine treatment ESI-MS spectrum of the mixture of 2-FPBA and mPEG-CA after 10 minutes. F) In the presence of designated small molecules, the monomer disappearance graph of (2-FPBA) in the reaction mixture.
[2-FBC hydrogel’s gelation, injectability, and self-healing ability]
Next, the author prepared 2-FBC hydrogel by mixing 4-arm PEG-CA polymer, 2-FPBA and PVA aqueous solution in a PBS solution at pH 7.4 (Figure 2A). The hydrogel is formed within 10 s, and its storage modulus increases with the increase of polymer concentration (Figure 2B). The hydrogel exhibits shear thinning and self-healing properties. When the shear stress exceeds 1000 Pa, G'is lower than G'', indicating that the 2-FBC hydrogel network ruptures under high stress (Figure 2C). When the shear rate was increased from 0.1 to 100 s-1, the viscosity of the hydrogel dropped sharply, indicating good shear thinning characteristics (Figure 2D-2E). When the hydrogel is strained at 500%, G'immediately drops to 0 Pa, and the hydrogel network is destroyed; when the strain turns to 1%, its modulus is completely restored within a few seconds (Figure 2F). The hydrogel can be lifted and bear a load of 3.37 g (Figure 2G). These results confirm that 2-FBC hydrogel has rapid gelation, excellent injectability and effective self-healing ability, which is conducive to wound healing.

Figure 2. Preparation and characterization of injectable self-healing hydrogels by 2-FPBA-mediated three-component condensation. A) Hydrogel formed by 4-arm PEG-CA, PVA and 2-FPBA. B) Storage modulus (Gʹ) and loss modulus (Gʺ) of 2-FBC hydrogel at different concentrations at 37°C. C) Shear of 10% 2-FBC hydrogel at 37°C Shear stress scanning test. D) Viscosity of 10% 2-FBC hydrogel. E) 10% 2-FBC hydrogel injectability. F) Evaluate 10% at room temperature with alternating strains of 1% and 500% 2 -Self-healing ability of FBC hydrogel. G) 2-FBC hydrogel self-healing process.
[Cysteine induces dissolution of 2-FBC hydrogel]
After 20 minutes of cysteine treatment, the 2-FBC hydrogel was completely dissolved (Figure 3B). Increasing the concentration of cysteine from 0 to 0.2 M resulted in an increase in the degradation rate and a decrease in the dissolution time from 1440 to 20 minutes. If adding glucose can accelerate the degradation, this may be due to the dissociation of the borate bond in the tandem dynamic covalent bond induced by glucose. The SEM image showed that after the solution, the pores of the hydrogel became smaller and more irregular, indicating that the hydrogel network was destroyed. Next, the author studied the on-demand dissolution characteristics of 2-FBC hydrogel on the skin surface (Figure 3H). The 2-FBC hydrogel was injected into pig skin, and then half of the hydrogel was covered with gauze soaked in cysteine. After 20 minutes, the part covered by the cysteine-soaked gauze was completely dissolved.

Figure 3. On-demand dissolution of 2-FBC hydrogel. A) Schematic diagram of 2-FBC hydrogel dissolution. B) Images of 2-FBC hydrogel before and after treatment with PBS (pH 7.4) or Cys solution (0.2 M, pH 7.4) for 20 minutes. C, D) Degradation of 2-FBC hydrogel in PBS (pH 7.4), PBS (pH 7.4) + 0.2 M glucose and PBS (pH 7.4) + cysteine. E) The dissolution time of 2-FBC hydrogel in PBS (pH 7.4) containing different concentrations of cysteine. F) SEM images of 2-FBC hydrogel before and after treatment with cysteine (0.2 m) for 10 minutes. G) Modulus changes of solutions (pH 7.4) exposed to biomolecules (glutathione, cystine, serine, histidine, glucose, lysine and cysteine). H) 2-FBC hydrogel adheres to pig skin tissue and is dissolved in cysteine soaked gauze as needed.
【2-FBC hydrogel promotes wound healing】
Next, the rat skin full-thickness defect model was used to evaluate the wound healing effect of 2‐FBC hydrogel. After 7 days of treatment, the wound closure rate of hydrogel treatment reached 73.5%. On the 15th day, no obvious wound was observed in the hydrogel treatment group. The wound healing effect of cysteine treatment is more attributable to cysteine's ability to reduce inflammation during dressing changes. In addition, hydrogel-treated wounds show fewer inflammatory cells, which may be due to the hydrogel wound dressing's ability to isolate the wound from the external environment. On the 7th day of treatment, all hydrogel treatment groups formed an epithelial layer, and after 15 days, more hair follicles and sebaceous glands were also observed, and denser collagen fibers were displayed at the incision.

Figure 4. Adhesion behavior of 2-FBC hydrogel and wound healing effect in vivo. A) After rinsing, the 2-FBC hydrogel adheres firmly to the rubber gloves and can withstand a load of 3.4 g. B) 2-FBC hydrogel can be formed. C) Schematic diagram of 2-FBC hydrogel wound treatment process. D) Use PBS (pH 7.4), commercially available wound dressing Prontosan, D-hydrogel (inject 2-FBC hydrogel into the wound area and then perform surgical excision) and R-hydrogel (hydrocoagulate 2-FBC) Glue is injected into the wound area and removed by cysteine-induced dissolution). F) H&E stained histological images of wound tissue in each treatment group. G) Masson stained histological image of skin tissue regeneration on the 15th day. H) Collagen content in different treatment groups based on Masson staining.
Conclusion: In short, the author has developed a series of new tandem dynamic covalent bonds composed of C=C double bonds composed of CKC reaction and borate esters, which are used to construct fast gelation and specific on-demand dissolution characteristics of cysteine Hydrogel dressing. The hydrogel is injectable, self-healing, and can be specifically degraded by cysteine by forming TzB complexes. When used as a wound dressing to treat full-thickness wounds, the hydrogel can quickly close the wound within seconds, and can also be dissolved as needed to change the dressing. This dynamic hydrogel opens up new ways for wound dressings and further expands the application of dynamic hydrogels based on Knoevenagel condensation reaction.
+86-18915413828(WhatsApp&WeChat)
Previous: China-Japan Friendship


