Zhang Jinzhong, University of California, Santa Cruz: Metal halide perovskites—from molecular clusters to magical size clusters and quantum dots

Recently, Professor Jin Zhong Zhang’s research group from the Department of Chemistry and Biochemistry at the University of California, Santa Cruz, studied the molecular clusters of metal halide perovskites (MHPs). , MCs), magic sized clusters (MSCs) and quantum dots (QDs) are intricately but interestingly interrelationships explored and provide important new perspectives.
Related work was published on Research (Research, 2021, 6047971) with the title "Interplay between Perovskite Magic-Sized Clusters and Amino Lead Halide Molecular Clusters".
Research Background
Crystal growth is an extremely important basic process in the field of materials science and engineering, which includes fascinating and complex nucleation and growth phenomena.
This also means that the crystal growth process is related to many factors, such as the initial phase of the sample, surface state, driving force intensity, temperature, pressure, and catalyst.
Compared with traditional bulk crystals, the growth of nanocrystals (NCs) or QDs usually requires the use of neutral or charged ionic molecular ligands for surface passivation for stability.
Therefore, the interaction between the ligand and the ion or atom in the sample precursor is particularly critical for the growth of NCs or QDs, but the current research and analysis of related mechanisms in this field are not thorough enough.
Among them, the A position is usually a monovalent inorganic cation (such as metal ions Cs+ and Rb+) or an organic cation (such as methylamine CH3NH3+, ethylamine CH3CH2NH3+, etc.); the B position is usually a divalent metal cation, such as Pb2+, Sn2+ and Ge2+ Etc.; X position is usually a monovalent halogen or pseudohalogen anion, such as I-, Br- and Cl-.
CH3NH3PbI3 and CsPbBr3 in the MHPs materials of CH3NH3PbI3 and CsPbBr3 have novel electrical, optical and magnetic properties, and have broad application prospects in the fields of photovoltaics (PV), light-emitting diodes (LEDs), sensors and quantum information technology.
Compared with traditional MHPs bulk crystals, perovskite quantum dots (PQDs) or perovskite nanocrystals (PNCs) have the optoelectronic properties with adjustable size and composition, such as light absorption and emission characteristics with broad application prospects.
Due to the adjustable composition and size of MPHs, the crystal growth process will be more complicated in nature. Especially for PQDs and PNCs, multiple ligands need to work together to achieve effective passivation of various surface defect states.
Research progress and prospects
Exploring the growth mechanism of PQDs or PNCs and the synergistic effect of related ligands has become one of the research hotspots in understanding the structural properties of perovskites.
In recent years, Professor Zhang Jinzhong’s research group has been committed to researching and optimizing the synergy between different ligands, proposed a "cocktail" passivation strategy, and actively seeks more in-depth analysis and exploration of the passivation mechanism.
The research group found that when using stronger (acidic or basic) ligands or higher concentrations of ligands, the reaction system can produce perovskite magic size clusters (PMSCs) instead of traditional PQDs.
Compared with PQDs, PMSCs have the advantages of narrower size distribution and control of the size and shape of nanoparticles with atomic precision.
Therefore, PMSCs are smaller in size than PQDs and are more uniformly distributed in solution, so they are more suitable for basic research on the evolution of basic properties of perovskite materials from molecular clusters to MSCs and QDs.
In addition, their research also found that under certain experimental conditions, by using appropriate ligands, the reaction can produce particles that are smaller in size and more difficult to absorb than PMSCs, even without the A (methylamine-MA+) component. It will also occur under conditions of participation.
Since particles smaller than PMSCs in theory have not formed a lattice structure like PQDs or PMSCs, and are more similar to molecules in structure, the particles are called molecular clusters (MCs).
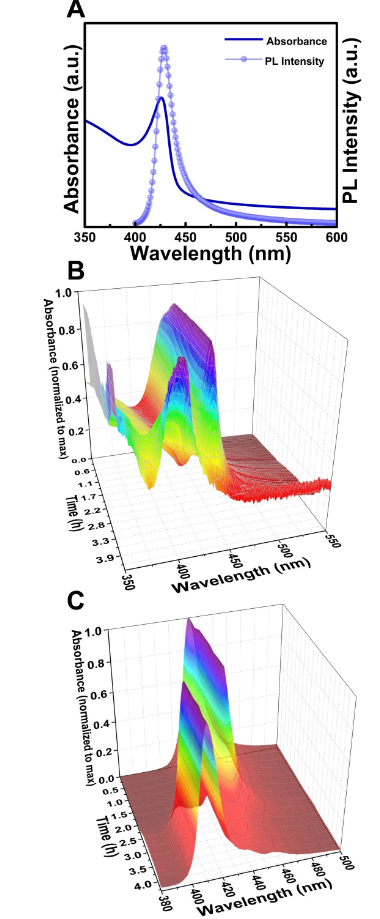
These MCs have ultraviolet absorption spectra and fluorescence emission spectra with a narrow half-width near ~400 nm (Figure 1), which is of great significance for applications that require blue light generation.
Figure 1 Optical characterization of MAPbBr3-VA-BTYA
In addition, in these MCs, butylamine (BTYA) ligand may play the role of methylamine component, but because BTYA is much larger in size than methylamine, BTYA cannot enter the crystal lattice and can only exist in MCs. surface.
Compared with MSCs, MCs are smaller in size and have disordered atomic arrangement.
Although the exact structure of MCs and MSCs is not yet clear, preliminary X-ray absorption studies will help to provide a preliminary understanding of the structure, and subsequent further experimental and theoretical studies will help clarify the structure and structure of MCs and MSCs. nature.
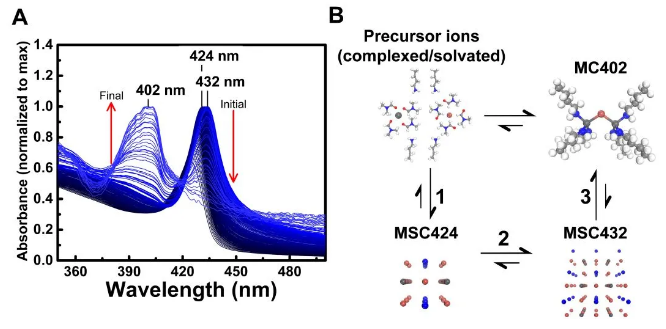
This study provides strong spectroscopic evidence for the existence of molecular clusters (MCs) and the dynamic transformation between magic size clusters (MSCs) and molecular clusters (MCs) (Figure 2).
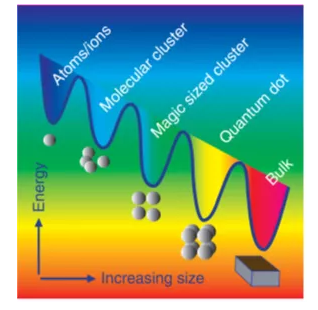
Figure 2 UV-Vis absorption spectrum changes during the conversion of MAPbBr3-VA-BTYA-PMSCs to PbBr2-BTYA-MCs (A); the relationship diagram of precursor ions, MCs and MSCs (B)
An interesting phenomenon was also found in the research. MCs seems to be more stable than MSCs under certain experimental conditions.
Generally speaking, MCs are the precursors to form MSCs, and then further produce QDs. In theory, MCs should be less stable than MSCs.
Of course, all these particles should be formed from atoms or ions in the form of solvation or complex structures (Figure 3).
image
Figure 3 Schematic diagram of different structures and related energy levels
This research advances a step toward an in-depth understanding of the crystal growth of MHPs. This research will help guide, design, and develop advanced optical materials in emerging technologies.
The first author of the paper is Evan T. Vickers, Ph.D., Department of Chemistry and Biochemistry, University of California, Santa Cruz, and the corresponding author is Professor Zhang Jinzhong
+86-18915413828(WhatsApp&WeChat)
Previous: Xuzhou Medical Univers


